Benefits of CMMS for the medical sector

Medical devices are tools that directly affect the health of their users. They involve considerable investments and high maintenance costs. It is therefore essential for structures equipped with them to have a program of GMAO adapted to medical sector, reliable and efficient.
Do you prefer to listen to this article? Discover it as a podcast here!
Maintenance in the medical sector
Everything health care facility must implement a maintenance program for its medical equipment. The complexity of this program depends on the size and type of the institution, its location, and the resources required. But the underlying principles of an effective maintenance program are always the same, regardless of the characteristics of the establishment.
Legal obligations
Maintenance is the responsibility of the operator as soon as the commissioning of medical equipment. In the event that the operator uses an external maintenance provider, it is recommended that their respective obligations and responsibilities be established by contract.
THEArticle R. 5212-25 of the CSP (Public Health Code) states that “the operator ensures the implementation of the maintenance and quality controls provided for the medical devices it operates. Maintenance is carried out either by the manufacturer or under his responsibility, or by a third-party maintenance provider, or by the operator himself.”
It is therefore up to him to define a maintenance policy for all the medical devices he uses, regardless of their class (I, IIa, IIb or III). While it seems obvious to implement a maintenance policy on so-called “critical” medical devices, the maintenance of class 1 and IIa medical equipment should not be neglected. A significant proportion of materiovigilance incidents whose identified cause is linked to a maintenance defect in fact concern this type of equipment, such as hoists or medical beds.
The medical devices concerned
The decree of 3 March 2003 establishes the list of medical devices subject to maintenance :
- medical devices required for the production and interpretation of diagnostic x-ray images;
- medical devices necessary for the definition, planning and delivery of radiation treatments;
- medical devices necessary for carrying out nuclear medicine procedures;
- medical devices for diagnostic or therapeutic purposes exposing people to ionizing radiation;
- class IIb and III medical devices resulting from the classification rules provided for in the decree of 20 April 2006 (amended by the decree of 15 March 2010).

For all these devices, the operator is required to apply the provisions provided for in article R. 5212-28 of the CSP, i.e.:
- to have an inventory of the devices it operates, kept regularly updated, listing for each of them the common and commercial names of the device, the name of its manufacturer and that of the supplier, the serial number of the device, its location and the date of its first commissioning;
- to define and implement an organization intended to ensure the execution of maintenance and internal or external quality control of the devices whose terms and conditions he specifies, which are transcribed in a document;
- to have information that makes it possible to assess the provisions adopted for the organization of maintenance and internal or external quality control as well as the methods of their execution;
- to implement the controls provided for in article R. 5212-27;
- to maintain, for each medical device, a register in which all maintenance and internal or external quality control operations are recorded;
- to allow access to the medical devices and information provided for in this article to any person in charge of maintenance and quality control operations.
La medical equipment maintenance can be divided into two main categories: inspection and preventive maintenance, and corrective maintenance.
Inspection and preventive maintenance
This category includes all activities scheduled in order to ensure the proper functioning of the equipment and to avoid breakdowns and failures. Performance and safety inspections are simple procedures whose purpose is to verify that a device is working properly and that it can be used safely.
La preventive maintenance corresponds to interventions scheduled in order to extend the life of a device and to avoid failures, in particular through the replacement of parts, calibration, lubrication or cleaning. An inspection can be carried out alone or combined with preventive maintenance operations. This can be relatively invasive, as it involves dismantling, cleaning or replacing certain components.
Corrective maintenance
The identification of a medical device failure occurs when a malfunction is reported by a user of the device, or during a preventive maintenance inspection. To get equipment back up and running as quickly as possible, quick and effective troubleshooting is required in order to understand the failure and determine its cause.
La corrective maintenance can be performed at the level of a component, a printed circuit or the medical device as a whole, which is then replaced. It is important to choose the level of maintenance adapted to each situation, based on the financial, material and human resources available, and the urgency of the need for repair.
The advantages of CMMS for the medical sector
In the medical sector as in all others, maintenance occupies a very important place in the activity of a structure. Hospitals, clinics, medical laboratories or even nursing homes have an interest in having a CMMS solution optimized to meet the many challenges they face.
Centralize and clarify information
Compared to paper documents or an Excel file, the use of a CMMS software for the medical sector makes it possible to clearly record information on maintenance tasks carried out. The consultation of the history of maintenance operations on medical equipment is thus easier and their use is simplified. Information loss and the risk of error are considerably reduced.
Save basic documentation and equipment
Basic documentation can be kept up to date using paper reports or a spreadsheet, but the use of a CMMS system facilitates the rational preservation of documents as well as the monitoring and improvement of performance, as indicated by the WHO.
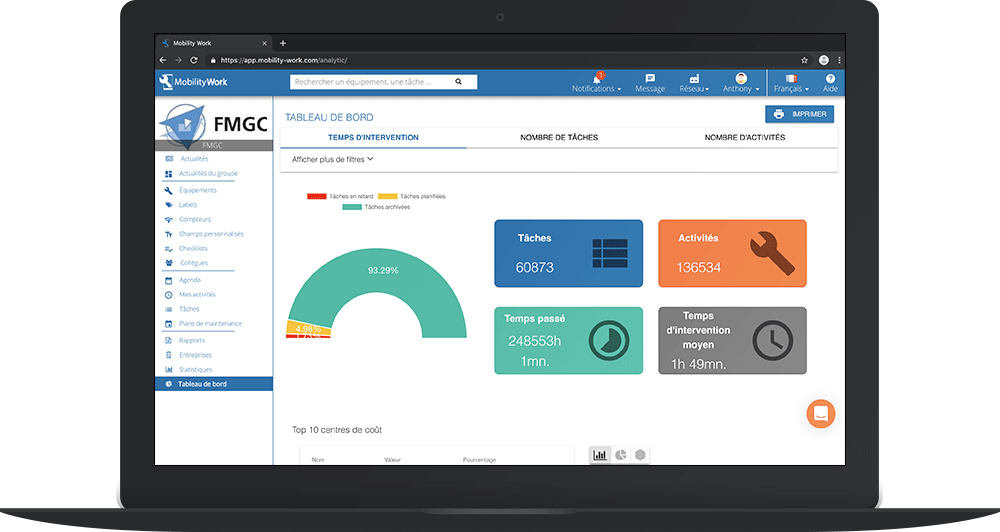
New-generation CMMS have tools to analyze all your maintenance data and monitor your performance
In addition, a CMMS software makes it possible to meet the rigorous requirements of maintenance in a medical environment thanks to the registration of all equipment and all equipment. It is thus possible to add photos, the location of each medical equipment in the structure, detailed sheets, etc. Thanks to this easily accessible data, the availability of the devices is significantly increased, which is essential in a medical or hospital environment.
Ensuring compliance with procedures
Having a complete history of all maintenance operations is essential, as only actions recorded in writing or digitally are legally recognized. The digitization of information thanks to the GMAO makes it possible to trace all the interventions carried out on equipment and to ensure that the controls imposed by law have been carried out. In addition, it allows a very quick extraction of the history in case of control. Finally, having an electronic signature feature allows technicians to validate interventions directly on site.
The benefits of 4.0 CMMS for the medical sector
The structures of medical sector often tend to turn to conventional and reputable software, which is however, in practice, very expensive and difficult to use. Les next generation solutions like Mobility Work are breaking with these outdated software, which is easy to use and learn.
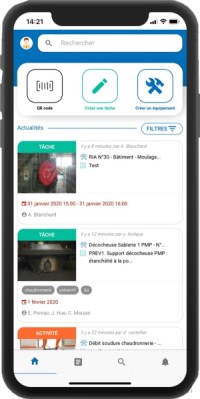
Mobility Work allows operators to be autonomous: calendar, checklists, creation of tasks, maintenance plans, notifications for each new activity...
This type of application allows better interconnectivity between services, in particular thanks to profiles adapted to services other than maintenance. The Purchasing Department, for example, can thus benefit from good visibility on maintenance activities and better anticipate reported needs. Orders are then placed more quickly, and procurement times and maintenance costs are thus reduced. This interconnectivity facilitates communication and collaboration between departments, and therefore the task of biomedical engineering, which is particularly in demand.
In addition, a mobile application such as Mobility Work, accessible at any time and from anywhere, allows for more reliable monitoring of interventions and reduces the risk of human error. Teams in charge of maintaining medical devices can in fact easily and quickly log their interventions in the application, access all the compliance documents they need and consult intervention indicators and reports at any time.
If the adoption of a GMAO is almost essential for establishments in the medical sector, choosing a next-generation solution makes it possible to overcome the shortcomings of traditional software while benefiting from the latest technological advances. La maintenance in the medical sector thus becomes easier and more reliable.
Any questions?
Contact us to discover the first CMMS that can be deployed in 3 weeks.