COVID-19: what CMMS to maintain medical equipment?

In the context of the health crisis that we are currently experiencing due to The Covid-19 pandemic, the maintenance of medical equipment is even more important than in normal times. Choosing the right one GMAO Allows you to adapt effectively both to health constraints and to the requirement of maximum reliability which weighs on the medical sector.
The maintenance of medical equipment in the time of Covid-19
La maintenance of medical equipment is subject to particularly strict regulations due to the considerable health challenges that weigh on the sector. The current global epidemic of Covid-19 reinforces these constraints even more.
Standards applicable to the maintenance of medical equipment
THEarticle R. 5212-25 of the Public Health Code states that maintenance is the responsibility of the operator as soon as the medical equipment is put into service: “the operator ensures the implementation of the maintenance and quality controls provided for the medical devices it operates. Maintenance is carried out either by the manufacturer or under his responsibility, or by a third-party maintenance provider, or by the operator himself.” It is therefore up to him to define a maintenance policy on all the medical equipment he uses, regardless of their class (I, Iia, IIb or III).
A decree of 3 March 2003 lists the medical devices concerned by the maintenance obligation:
- class IIb and III medical devices resulting from the classification rules provided for in the decree of 20 April 2006 (amended by the decree of 15 March 2010).
- medical devices necessary for the definition, planning and delivery of radiation treatments;
- medical devices required for the production and interpretation of diagnostic x-ray images;
- medical devices for diagnostic or therapeutic purposes exposing people to ionizing radiation;
- medical devices necessary for carrying out nuclear medicine procedures;
The CFR 21 Part 11 standard
La CFR Part 11 standard concerns the maintenance of machines and equipment in the pharmaceutical, medical and food sectors mainly. Issued by the American FDA (Food and Drug Administration), it is mandatory for all companies that market products in the US. In particular, this standard requires compliance with strict processes for entering and archiving data. It is built around two main axes:
- the electronic signature, which aims to certify critical information or documents and, possibly, to comment on them;
- the audit trail, whose objective is to keep track of any action or modification that has occurred on the data or on the structure.
La Standard 21 CFR Part 11 therefore requires strict and advanced management of access and user rights, a log of connections and accesses as well as a precise definition of the hierarchical schemes applicable to the validation of the most sensitive data.
Maintaining medical equipment during the Covid-19 epidemic
During the pandemic of Covid-19, maintain medical equipment is both more important and more complex. On the one hand, they need to be cleaned even more frequently than usual. On the other hand, the sustained activity of certain medical sectors at the height of the health crisis, and the resumption of activity at a steady pace in others with the end of lockdown, requires particularly rigorous and effective maintenance, in order to ensure the reliability of equipment while disrupting its use as little as possible. Finally, the functioning of teams in small numbers complicates their work and that of the managers responsible for organizing interventions.
Choosing the right CMMS software to maintain medical equipment
Even more than usual, choose a CMMS software adapted to the medical sector and to health crisis makes it possible to ensure a optimal maintenance of medical equipment.
CMMS, an essential tool in the medical sector
The standards that apply to the maintenance of medical equipment, and in particular the Standard 21 CFR Part 11, make it almost mandatory to have a maintenance management platform such as CMMS. In fact, it makes it possible to clearly record information on the tasks performed. In addition, consulting the history of interventions is easier. Finally, the loss of information and the risk of errors are greatly reduced.
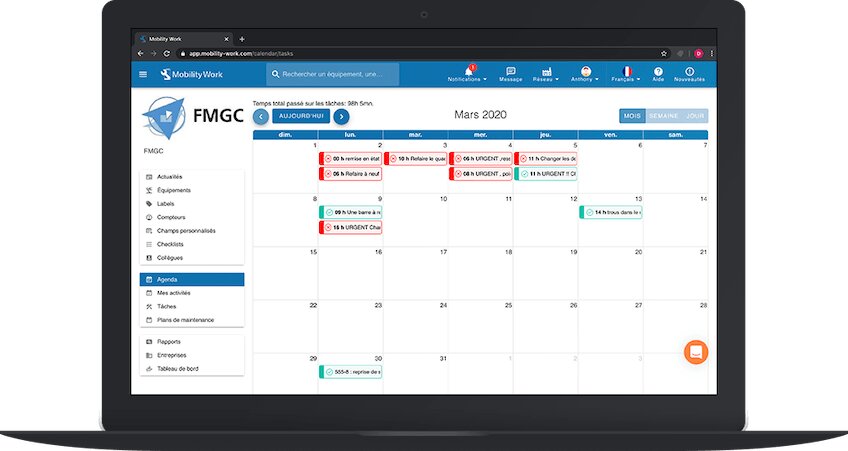
However, old-generation CMMS solutions are often not adapted to the medical sector and to the particularly strict standards that weigh on it. Their use is therefore complex and unintuitive, which poses a serious problem when all operations must be carefully recorded, and slows down the work of teams.
The new CMMS at the service of compliance with standards
One next-generation CMMS solution such as Mobility Work represents a major advance for the maintenance of medical equipment in compliance with standards such as 21 CFR Part 11. Designed on the model of everyday applications, it is, for example, very easy to use. It allows users to enter and consult data and information with great ease. This promotes strict compliance with standards by the teams responsible for maintaining medical equipment, whose every intervention is thus meticulously recorded.
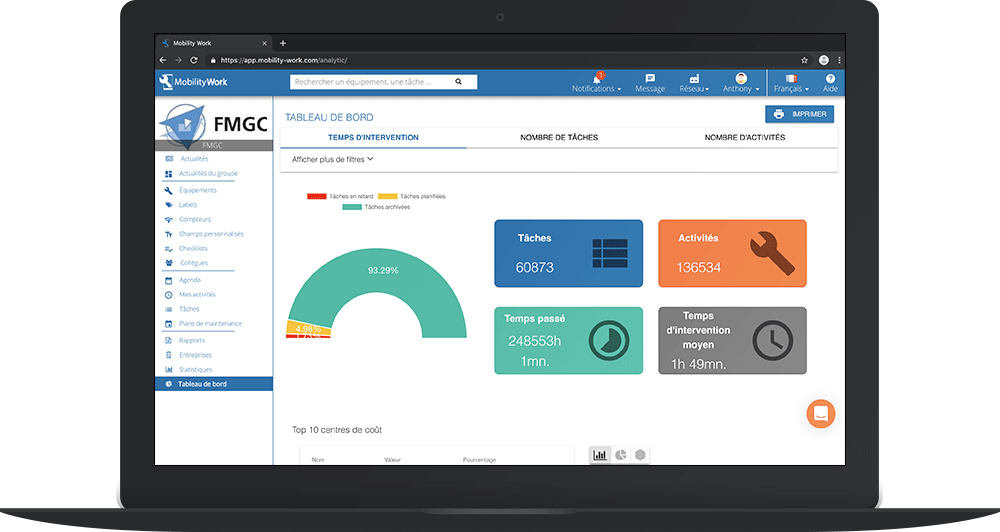
In addition, thanks to its numerous functionalities, the Mobility Work application makes it possible to conduct an in-depth analysis of the equipment maintenance strategy. It also makes it possible to meet maintenance audits very simply, and to meet the requirements of Standard 21 CFR Part 11 on the subject.
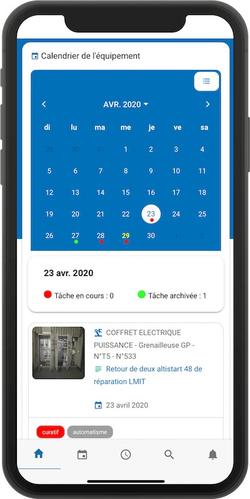
Adapting to health restrictions thanks to mobile CMMS
In addition, the Next generation CMMS, also called “CMMS 4.0”, is mobile. This means that such an application can be used from any connected mobile device (smartphone, tablet, laptop...). In times of strong health constraints and frequent teleworking, this represents a major asset.
Indeed, both maintenance teams and managers can consult all the documents and information they need at any time. For the former, this allows them, for example, to have access to the recommendations of equipment manufacturers and to the processes imposed by the standards during their interventions. For the latter, this makes it much easier to analyze the maintenance strategy and adapt it to the situation. The management of small staff and required to comply with exceptional health standards is also facilitated.
The maintenance of medical equipment is subject to numerous constraints. Some, like 21 CFR Part 11, are structural. Others, such as health measures to combat the Covid-19 epidemic, are circumstantial.
To meet these major challenges, it is crucial to choose a suitable and effective CMMS solution. Thanks to Mobility Work, compliance with all standards is easier to implement, and maintenance becomes at the same time more rigorous, more efficient and easier.
Any questions?
Contact us to discover the first CMMS that can be deployed in 3 weeks.